
SPONSORS & CROS
Clinitiative specializes in aligning with sponsors and CROs to provide our network of exceptional clinical trial sites to increase the success rate of their clinical research programs.
We have developed a clinical research site network that is focused on diversity, quality and integrity. Our sponsor and CRO partners recognize the value of Clinitiative’s sites starting from feasibility through study closeout. This affords Clinitiative the unique opportunity to continue collaborations on new and exciting clinical programs.
Sponsor & CRO Solutions
SiteSelect™
Site selection and feasibility management for internal and external sites
ClinReview™
Real-world protocol review from reputable principal investigators
ClinDefense™
Bid Defense Support From Our Expert Key Opinion Leader Network
ClinConsult™
Consulting Solutions For Sponsor & CROs
ClinFirst™
A Partnership Program offered at flexible levels to meet the needs of sponsors and CROs of any size, with any study volume
ClinFirst™
A Partnership Program offered at flexible levels to meet the needs of sponsors and CROs of any size, with any study volume
ClinFirst offers opportunities for…
A multi-year alliance agreement and master CDA to enable continuous alignment of sponsor and CRO pipelines with Clinititiave clients on a monthly, quarterly, or as needed basis (Partnerhsip terms can vary as needed)
Get on-demand study metrics for pre-award studies to assist with bid defense
Centralization for study CDAs and feasibilities, startup regulatory and study budgets and contracts (for participating developed sites)
Protocol consultations with Clinitiative KOL
ClinFirst is offered in three flexible tiers— we can add or edit these offerings as needed.
Our Site Network
Average diversity across all sites

- African American 21%
- Asian
6% - Native American
1% - Native Hawaiian
<1% - White
39% - Hispanic/Latino
31% - Other
3%
20+
Average Years of Experience
15+
Phase 1 Units
5+
Hospital Affiliations
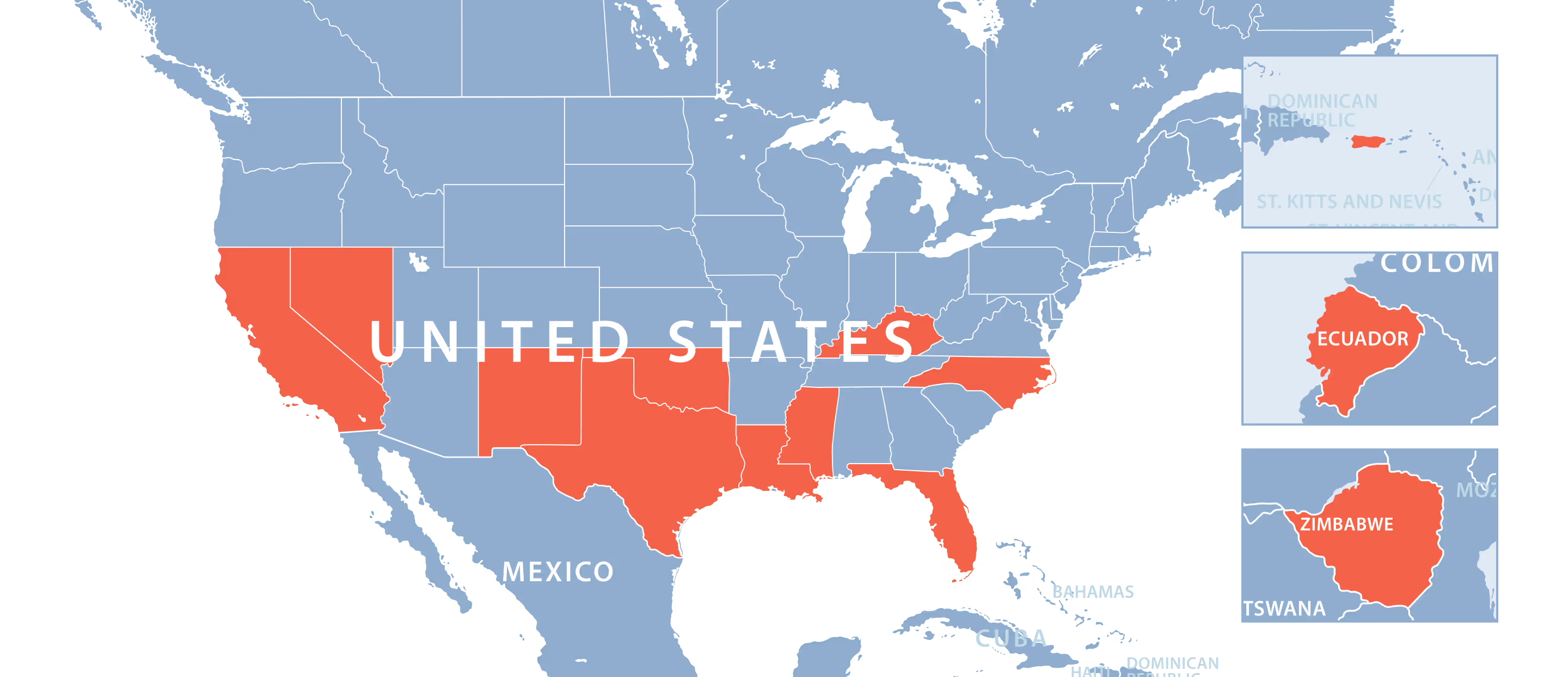
We’ve vetted the most experienced and highest qualified clinical sites for your next clinical trial
To qualify as a Clinitiative site client we consider:
Company longevity, capabilities, capacity
Locations
Previous study performance metrics
Diversity metrics and diversity plans
Company culture
483 notices/resolutions
Principal investigator background, reputation, experience, license good-standing
Quality site SOPs for: training, issue escalation, informed consent, data management /
documentation, recruitment methods, specimen handling/safety, AE/PD, etc.
documentation, recruitment methods, specimen handling/safety, AE/PD, etc.
Staff composition, training, equipment, and study experience
Responsiveness, transparency, dependability, excellence
Patient recruitment and retention history, issue mitigation plans
Unique enrollment techniques, capabilities
Once a site is in our network, we continue to monitor quality by…
Meeting with sites on a weekly basis to discuss current and upcoming trials
Meeting with sponsor & CRO partners on a weekly basis to discuss our sites’ progress within awarded studies
Maintaining access to our sites’ Clinical Trial Management (CTMs) to monitor ongoing trials






